

This negative regulation leads to high levels of glycogen synthase activity during fasting conditions, which in turn increases substrate (futile) cycling, priming the system for a rapid response once an external source of glucose is restored. Analysis of the model reveals a critical role for the inhibition of glycogen synthase phosphatase by glycogen phosphorylase a. The model qualitatively reproduced physiological changes that occur during transition from the fed to the fasted state. The model was validated against previous experimental results concerning glycogen phosphorylase a (active) and glycogen synthase a dynamics. Here we use computational modeling to explore hepatic glycogen regulation under fed and fasting conditions in the context of a whole-body model. The integration of hepatic glycogen regulation with extra-hepatic energetics is a key aspect of these adaptive mechanisms. These transitions necessitate major metabolic changes to maintain energy homeostasis as the source of blood glucose moves away from ingested carbohydrates, through hepatic glycogen stores, towards gluconeogenesis.
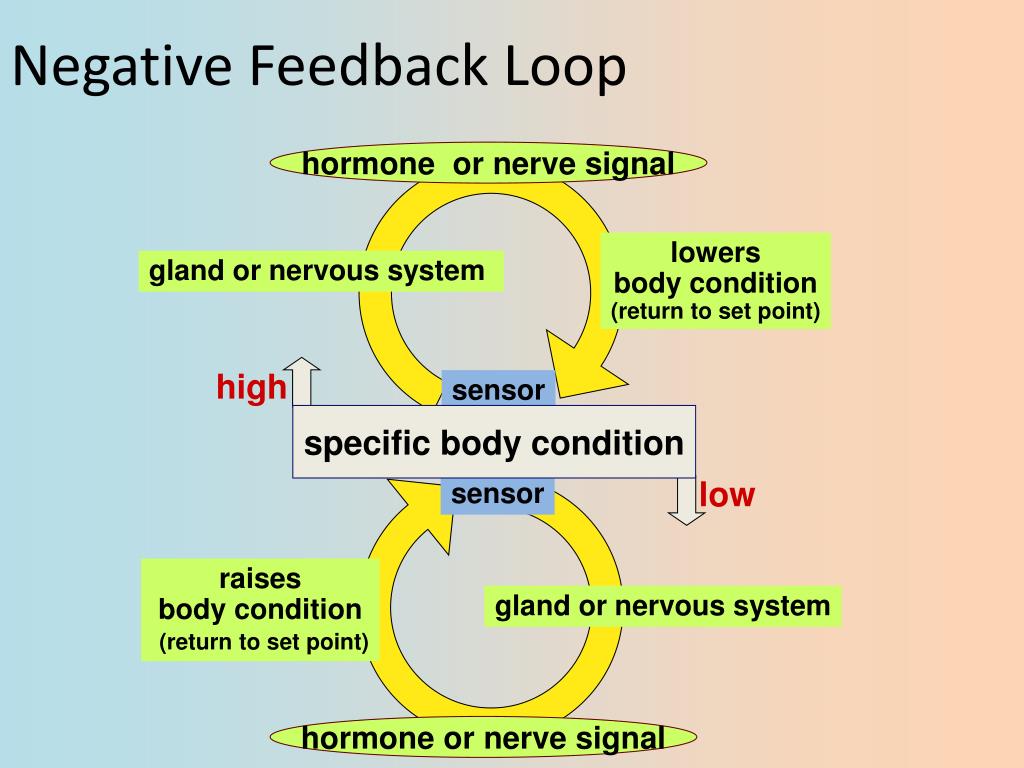
When the furnace produces enough heat to elevate temperature above the set point of the thermostat, the thermostat is triggered and shuts off the furnace (heat is feeding back negatively on the source of heat)Ī Whole-body Model For Glycogen Regulation Reveals A Critical Role For Substrate Cycling In Maintaining Blood Glucose HomeostasisĪbstract Timely, and sometimes rapid, metabolic adaptation to changes in food supply is critical for survival as an organism moves from the fasted to the fed state, and vice versa. The heating system in your home is a simple negative feedback circuit. Negative feedback is seen when the output of a pathway inhibits inputs to the pathway. Instances of positive feedback certainly occur, but negative feedback is much more common. Feedback Control of Hormone Production Feedback circuits are at the root of most control mechanisms in physiology, and are particularly prominent in the endocrine system. Shutting off secretion of a hormone that has a very short halflife causes circulating hormone concentration to plummet, but if a hormone's biological halflife is long, effective concentrations persist for some time after secretion ceases. Rate of degradation and elimination: Hormones, like all biomolecules, have characteristic rates of decay, and are metabolized and excreted from the body through several routes. Rate of delivery: An example of this effect is blood flow to a target organ or group of target cells - high blood flow delivers more hormone than low blood flow. Such control is mediated by positive and negative feedback circuits, as described below in more detail. The concentration of hormone as seen by target cells is determined by three factors: Rate of production: Synthesis and secretion of hormones are the most highly regulated aspect of endocrine control. Almost inevitably, disease results when hormone concentrations are either too high or too low, and precise control over circulating concentrations of hormones is therefore crucial.
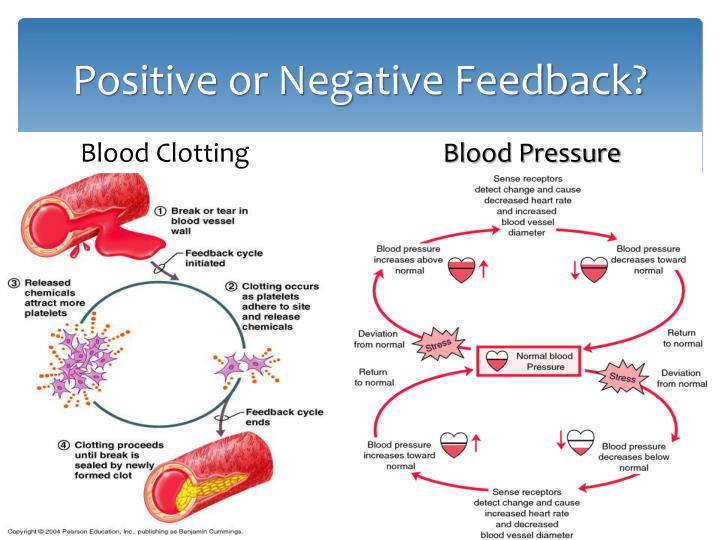
The physiologic effects of hormones depend largely on their concentration in blood and extracellular fluid.


 0 kommentar(er)
0 kommentar(er)
